
We also examine several methods of compensating for cyclable lithium loss associated with passive-film formation and calculate the effect more » each has on a cell's specific energy. We define irreversible capacity loss as that associated with active material loss and define two types of reversible capacity loss associated with balanced and unbalanced side reactions. We attempt to clarify what is meant by capacity loss and cyclable lithium loss by defining these terms in the context of electrode state-of-charge restrictions. In lithium-ion cells, there are several different classes of capacity loss, both reversible and irreversible, that limit the cell's exploitable specific capacity and can lead to eventual cell failure. The insights gained will prove invaluable to the development of strategies for extending the cycle life of energy-dense Li–S batteries.
#Anode and cathode reaction calculator full
The use of an anode-free full cell configuration provides a framework for accurate assessment of the dynamics of lithium inventory depletion and characterization of the accompanying interphasial evolution with cycling. This indicates considerable gas evolution and is also correlated with the loss of lithium inventory. The bulk of the deposited lithium is shown to be composed of various fully reduced interphasial components, including several hydrogen-containing species that show a substantial reduction in intensity with cycling. The trapped metallic lithium is rendered electrochemically inactive by the growth of a resistive electrolyte decomposition interphase on the lithium surface. Time-of-flight secondary ion mass spectrometry measurements on the deposited lithium reveal the presence of substantial metallic lithium even after most of the active lithium inventory has been depleted. Lithium inventory loss is shown to be the main factor limiting the more » overall cyclability of Li–S batteries. We use here an anode-free full cell configuration, pairing a Li 2S cathode with a bare nickel current collector with no lithium metal on it, to quantitatively estimate the lithium inventory loss per cycle. An in-depth understanding of the dynamical behavior in liquid electrolytes, including the mechanisms underlying depletion of lithium inventory and evolution of lithium interphases, is crucial to make Li–S batteries a reality. The promise of high energy density lithium–sulfur batteries with long cycle life is currently tempered by the rapid degradation of lithium-metal anodes with cycling. These conclusions are presented in terms of lithium-ion batteries, but this framework may be extended to describe other battery = , Experiments are presented to support the framework and demonstrate that cells can transition between limitation types. CE and CR depend on cell limitation type and are increased by faster cycling. From the framework, CE depends on either the oxidation side-reaction current or the reduction side-reaction current, but not both. Important conclusions regarding cyclable lithium inventory, limitation type, and the interpretation of CE and CR are discussed. The otherwise unmeasurable oxidation and reduction side-reaction currents are expressed in terms of cycling current, CE, and CR for both limitation types.
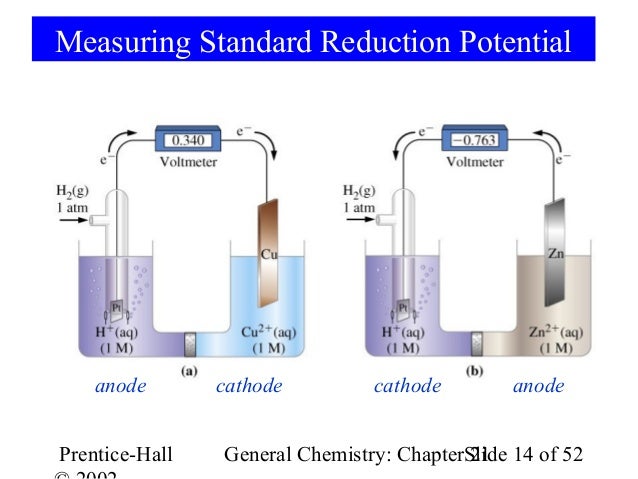
A cyclable lithium inventory (CLI) framework is developed to explain the fundamental differences between inventory-limited and site-limited cells.

Here, the battery performance metrics of Coulombic efficiency (CE) and capacity retention (CR) are derived in terms of cycling current and side-reaction currents at each electrode.


 0 kommentar(er)
0 kommentar(er)
